
12+ N2 Molecular Orbital Diagram Robhosking Diagram
Lewis Dot Structure The Lewis structure indicates the atom and its position in the model of the molecule using its chemical symbol. It also describes the chemical bonding between atoms present in the molecule. Mainly, the structure depicts the arrangement of the valence shell electrons of an element.

89. Covalent Bonding(35) MOT(10) Nitrogen molecule. Madoverchemistry
STEP 3. Fill molecular orbitals using energy and bonding properties of the overlapping atomic orbitals. Keep in mind the energy of the atomic orbitals and molecular orbitals! The following factors contribute to the position of one MO with respect to other MOs. More nodes = more energetic = higher MOs.

a) Simplified N2 orbital diagram. Reproduced with permission.104... Download Scientific Diagram
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) N2 Molecular Orbital Diagram. N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules.

How to draw the Molecular orbital diagram of N2 Molecular Orbital Theory Diagram Chemistry
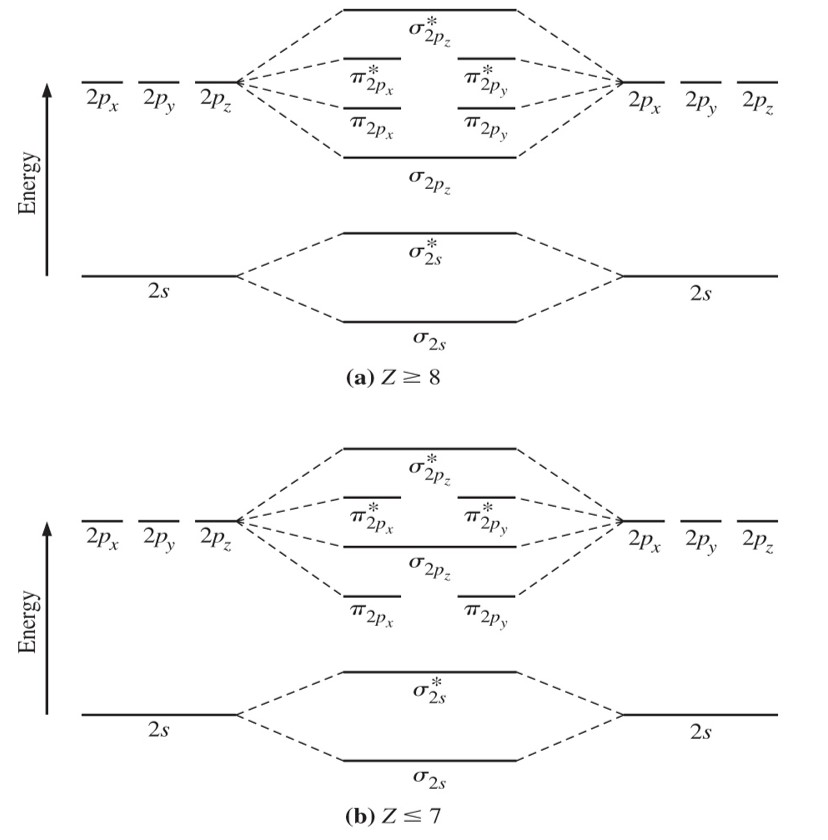
If we build the MO diagram for N2, it looks like this: First though, notice that the p orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be. Anyways, for the electron configurations, you would use a notation like the above.
12+ N2 Molecular Orbital Diagram Robhosking Diagram
Orbital Overlap Diagram for N2 chemistNATE 247K subscribers Subscribe 318 16K views 2 years ago Here, we draw the orbitals that overlap to hold together a molecule of N2, nitrogen gas,.

draw the molecular orbital diagram of O2 or N2 Brainly.in
Dihydrogen (H 2). To generate the molecular orbital diagram for H 2, begin with the valence atomic orbitals.In the case of H 2, each H atom has a single 1s valence orbital.As seen previously, then two s orbitals overlap they form a σ bonding combination and a σ* antibonding combination.The σ bonding molecular orbital increases the electron density between the two H atoms and has no nodes.

12+ N2 Molecular Orbital Diagram Robhosking Diagram
Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +.Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals.
N2 Molecular Orbital Diagram Free Diagram For Student
A molecule must have as many molecular orbitals as there are atomic orbitals. Figure 9.7.1 9.7. 1: Molecular Orbitals for the H 2 Molecule. (a) This diagram shows the formation of a bonding σ 1s molecular orbital for H 2 as the sum of the wave functions (Ψ) of two H 1 s atomic orbitals.

N2 Molecular Orbital Diagram Free Wiring Diagram
Molecular orbital diagram: The molecular orbital diagram describes the chemical bonding in a molecule based on molecular orbital theory (MOT) and linear combination of atomic orbital (LCAO). The molecular orbital diagram has molecular orbital energy level at centre and is surrounded by atomic orbital energy level.
Molecular Orbital Diagram For N2 Diagram For You
Figure 9.8.4: Molecular Orbital Energy-Level Diagram for a Heteronuclear Diatomic Molecule AB, Where χ B > χ A. The bonding molecular orbitals are closer in energy to the atomic orbitals of the more electronegative B atom. Consequently, the electrons in the bonding orbitals are not shared equally between the two atoms.

In the formation of N2^+ from N2, the electron is removed from
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory..
Solved Chapter 9 Problem 91AE Solution Masteringchemistryplus For Chemistry 11th Edition
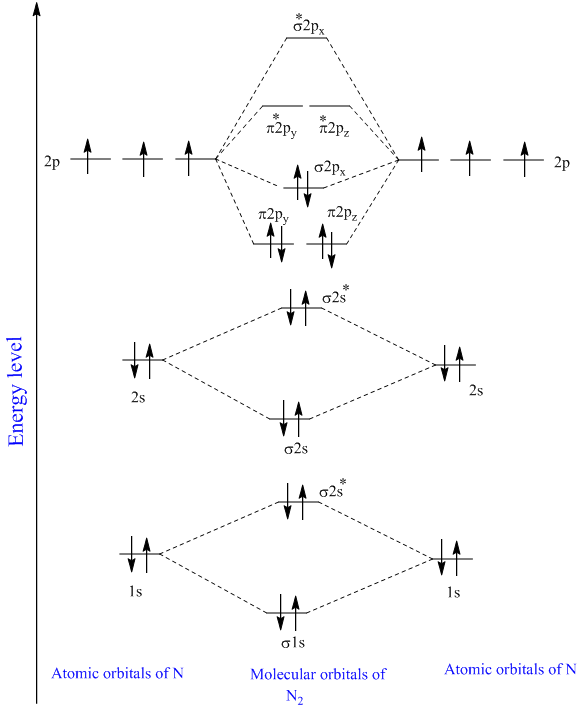
The molecular orbital diagram for N2 shows that there are two electrons in the sigma bonding molecular orbital, which results in a stable double bond between the nitrogen atoms. The sigma antibonding orbital remains unoccupied. This diagram indicates that N2 has a triple bond with a strong bond strength due to the stable sigma bonding molecular.

(a) N atom orbitals and their linear combination to form N2 molecular... Download Scientific
To obtain the molecular orbital energy-level diagram for \(\ce{O2}\), we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure 9.10.1 . We again fill the orbitals according to Hund's rules and the Pauli principle, beginning with the orbital that is lowest in energy.

Draw molecular orbital energy level diagram for N2 molecule and calculate it's bond order
Molecular Orbitals for N2 Jmol models of calculated wavefunctions To view a model, click in the circle of a molecular orbital in the energy level correlation diagram shown Ignore any popup warning and click on the green Continue button which appears Mouse Control of Models

mo energy diagram for n2 Cosleek
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.. Between [latex]\ce{N2}[/latex] and [latex]\ce{O2}[/latex], the order of the.

Draw The Orbital Diagram alternator
1. Write down the electronic configuration of N2 atoms N 2 is composed of two nitrogen (N) atoms. The electronic configuration of each N-atom is 1s2 2s2 2px1 2py1 2pz1. Usually, only the valence electrons are displayed in the MO diagram of a molecule, therefore, it is important to note that each N-atom contains 5 valence electrons.